Risk Partners Life Sciences Roundtable 2025, thank you very much!
Risk Partners Life Sciences Roundtable 2025, thank you very much!
How to properly insure clinical trials to protect subjects and satisfy the ethics committee from your leading independent provider Risk Partners.
Clinical trials are required to test non-approved drugs or new medical devices. The clinical trial insurance discussed on this page is mandatory and serves to protect the trial participants, irrespective of any contractual or tortious basis for liability. The insurance is taken out in favor of the trial participant with a special insurer approved within the scope of the German Medicinal Products Act (AMG) or Medical Devices Implementation Act (MPDG) or Medical Device Regulation (MPG) - usually with the help of a special insurance broker such as us as your risk partners.
In contrast, it is not mandatory to take out clinical trial insurance if it is not a clinical trial to test a medical device or a medicinal product. Therefore, scientific studies or clinical trials of food supplements, for example, which are often carried out for marketing purposes, are not subject to compulsory insurance. However, voluntary insurance to protect the test subjects should be considered.
This is due to the Declaration of Helsinki. In 1964, the World Medical Association committed itself for the first time to strict compliance with ethical standards in medical research in a self-declaration. Principles:
A challenge for governments around the world
In Germany, insurance for test persons has been mandatory since 1978. Across Europe, corresponding laws were only introduced after 1985(introduction of the EU directive on product liability).
Clinical trial insurance must cover damage caused by the conduct of a study or other clinical trials in which a person is killed or physically injured or their health is impaired in any way. The insurance must also provide benefits in cases where no one else is responsible for the damage. The main promise of clinical trial insurance to trial subjects applies, for example, in the event of permanent disability or death.
Sponsors of clinical trials for medicinal products or medical devices are required by law to provide evidence of clinical trial insurance in order to obtain official approval to conduct the trial. Ethics commissions therefore also prescribe a corresponding insurance policy for trial subjects. For other types of clinical trials for which there is no statutory insurance requirement (such as new examination and treatment methods), many sponsors also take out appropriate insurance to protect the test subjects. We are also increasingly seeing that a corresponding promise of insurance can be helpful in recruiting trial subjects and that voluntary benefits such as travel accident insurance for trial subjects on the way to and from the study center are now commonplace.
Possible additional cover
From a risk management perspective, it is essential to create a common risk awareness in the first step, which is largely supported by the risk strategy. Convince your investors in the due diligence process of your next financing round with a risk report and a deep understanding of your risks, thereby safeguarding against risks that could threaten your company's existence.
On the basis of sound risk and insurance management, we develop a decision-making basis for your company. You decide what to insure and what not to insure. Because not every risk is worth insuring. Here is our basic approach:
Your added value through active risk and insurance management:
The importance of this for young growth companies was demonstrated not least by the Silicon Valley Bank case, which was identified as part of a risk management process and - if necessary - would have led to an adjustment of the banking/treasury strategy.
If a clinical trial falls under the German Medicinal Products Act (AMG), according to § 40a General requirements for the clinical trial.
Beyond the requirements of Regulation (EU) No. 536/2014, a clinical trial may only be conducted as long as:
in the event that a human being is killed or the body or health of a human being is injured during the conduct of the clinical trial, insurance exists which also provides benefits if no one else is liable for the damage, in accordance with the following provisions:
What many companies only become aware of in discussions with the notified bodies is the obligation to provide insurance in accordance with MDR Article 10, Paragraph 16 (2), which is worded as follows:
"Manufacturers shall take precautions appropriate to the class of risk, the nature of the product and the size of the undertaking to ensure adequate financial coverage of their potential liability under Directive 85/374/EEC, without prejudice to more stringent protective measures under national law."
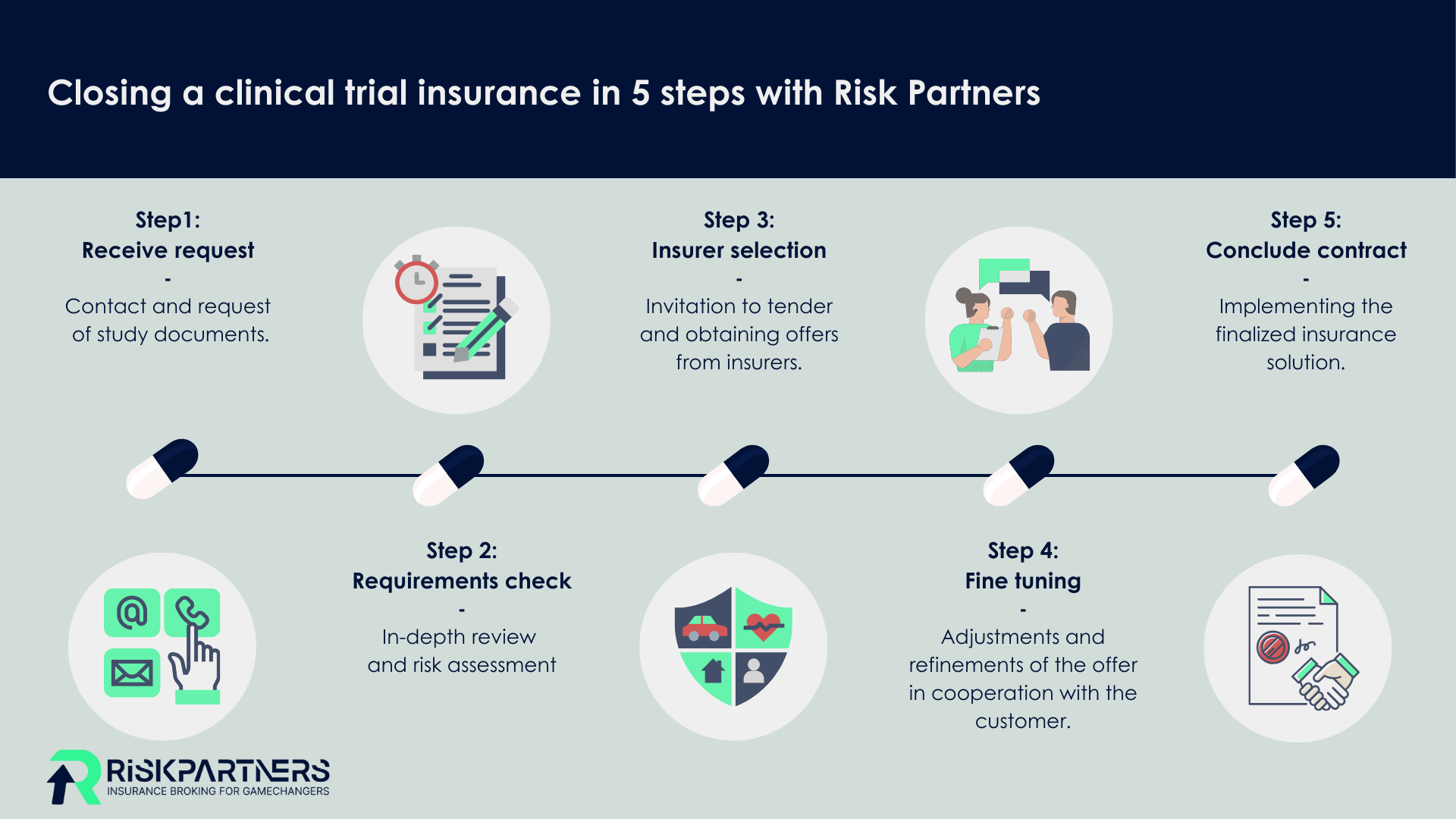
"Through the partnership with our subsidiary "Atrialis GmbH - experts in clinical trial insurance", we are one of the leading providers in the market. Thanks to our many years of experience, we offer you competent support in the risk structuring and risk transfer of complex international trials as well as in the event of claims for clinical trial insurance."
Jutta Zaglauer - Senior Risk Advisor Life Sciences

The insurance premium for clinical trial insurance cannot be quantified across the board, but is usually between 30 and 400 euros per subject. Pure screening subjects are usually included without a premium, as is the placebo cohort. The following criteria are taken into account in the insurer's risk assessment:
The last point can become very important, for example, if you have to cover the very high sums insured required by the German Ethics Commission for a particularly risky study project (see also the following section on the special features of international studies). This is where a national provider can reach its limits - in case of doubt, even if it continues to purchase capacity from its (expensive) reinsurers. We discuss this problem, which is particularly common in the insurance of multinational clinical trials, with you at an early stage after we have studied the trial project, as experience has shown that, in addition to the fulfillment of requirements, an insufficient sum insured can become a problem at the end of the trial. In order to avoid uneconomical emergency solutions, we structure this for you at the outset, e.g. by adding 2-3 insurers (keyword: co-insurance & excess liability insurance).
In certain countries, such as the USA, we are seeing a trend of trial sites demanding a higher limit in clinical trial insurance than the pure regulatory requirements of ethics and legislation. In the USA, for example, a USD 5 million limit is sufficient by law, but some trial sites demand a limit of at least USD 10 million per claim and in aggregate as a total amount. This serves to extend their protection against potential liability cases, which can also exceed the regulatory framework. There is no (liability) limit due to the statutory minimum requirements. The solution can be a voluntarily higher sum insured for individual countries or study centers, e.g. in order to win them over in the event of increased recruitment costs and to meet their requirements for participation in the study. Umbrella cover is usually installed for a clinical trial insurance policy, which, like an umbrella, offers additional, comprehensive protection if the preceding basic cover of the clinical trial insurance is exhausted.
This can also be the case with an extended reporting period (ERP), for example, where an umbrella insurance policy extends the cover in accordance with the desired or required term. The risk carrier of the umbrella cover is usually different from the insurer of the underlying clinical trial insurance. This means that limits and premiums, as well as cover contents (e.g. term of the ERP) can be negotiated better and more attractively, as "weaknesses" in the clinical trial insurance can be closed and negotiated with the umbrella solution.
Advantages of an umbrella solution for the study sponsor:
Studies in several countries must comply with local laws and regulations, required sums insured and the issuing of policies in the local language, which is why country-specific policies are necessary. This starts with different legal standards and lower or higher prescribed sums insured per test subject. Local policies can also be used to meet the requirements of statutory subsequent liability periods and the special feature that all participants in the study must be able to read the insurance policy in their local language.
It is also advisable to provide a no-fault indemnity for volunteers to ensure the required insurance coverage according to local laws. This no-fault insurance is required by law in many countries where you may be conducting your study.
Or would you have thought that the maximum compensation per test subject is subject to an extreme range within the EU member states? We are therefore seeing an increasing trend towards additional umbrella cover, particularly for multinational studies, in order to make insurance cover uniform, transparent and risk-adequate. For example, the statutory limit can vary significantly from EUR 29,000 to EUR 5,000,000 per subject in other countries. Umbrella cover is therefore available for all study countries and offers protection if the local minimum requirements for clinical trial insurance have been exhausted.
We are familiar with these challenges and, thanks to our many years of intensive contact with leading insurance companies from international projects in almost every country in the world, we can offer you a solution for clinical trial insurance. Even with the most important insurance market in the world Lloyd's of London in order to organize special studies and higher levels of cover if required. Last but not least, we recently joined forces with our experienced colleagues at Atrialis GmbH - experts in clinical trial insurance (formerly Aric Advances Risk & Insurance Consulting, known for its "aric trial insurance approach", among other things) and pool our experience. In future, this will also be reflected in the addition of a medical expert to the team, who we will involve accordingly in the event of a claim.

There is a clear trend in the world of digital health solutions: more and more innovative apps and digital applications are being approved as medical devices. In particular, so-called DiGA (digital health applications) offer great benefits by improving care and making treatments more efficient. However, as with traditional medical devices, DiGAs also require careful testing to ensure safety and efficacy. And this is precisely where clinical trial insurance comes into play.
Why is clinical trial insurance important for DiGA?
Digital health applications are classified as medical devices under the Medical Device Regulation (MDR). These applications intervene in the diagnostic or therapeutic process and therefore harbor potential risks - from incorrect diagnoses to undesirable therapeutic effects.
Before a DiGa is approved, comprehensive testing by the Federal Institute forDrugs and Medical Devices (BfArM) is required. Clinical studies are essential to prove efficacy and safety. During these studies, the protection of the trial subjects must be the top priority. Clinical trial insurance offers indispensable protection here, which is not only ethically necessary but also mandatory by ethics committees.
Clinical trial insurance offers study participants the necessary protection against potential risks that may arise when using such digital applications - especially when it comes to misuse or undesirable side effects. At the same time, it minimizes the liability risk for providers, who are legally obliged to take comprehensive precautions in accordance with the MDR. For the providers of DiGA who test these applications in clinical trials, this means that they are obliged to take out clinical trial insurance to protect themselves against liability claims. This insurance ensures that study participants are protected in the event of damage to health or adverse effects during the study.
How does clinical trial insurance for DiGA work?
Customized clinical trial insurance offers comprehensive protection for all participants in a clinical trial:
The insurance covers possible health consequences of a misdiagnosis or therapy that could arise from the use of the DiGA. This is particularly important for health apps that intervene directly in the diagnostic or therapeutic process. The insurance protects not only the test subjects, but also the manufacturers and providers of such digital health solutions. Especially when DiGA is approved by the BfArM, the responsibility for the safety and success of the application is high. Clear protection through clinical trial insurance ensures that all parties are covered in the event of adverse events.
The new Medical Device Regulation (MDR) provides for a "quasi-insurance obligation" for medical devices such as DiGA. According to Article 10 of the MDR, manufacturers of medical devices must take precautions to cover their liability financially. For DiGA, this means that both product liability insurance and clinical trial insurance are essential.
➔ Conclusion: Security and trust in digital health applications
Clinical trial insurance is an indispensable component of any clinical study that validates a DiGA as a medical device. It not only ensures the protection of the trial subjects, but also creates a solid basis for the legal and financial security of the providers.
In view of the increasing importance of DiGA - as a reimbursable benefit under statutory health insurance - comprehensive cover is becoming ever more important. Our team of experts at Risk Partners is on hand to provide you with the best possible protection for your digital health application with individually tailored solutions.
Do you also need support in securing your digital health application or clinical trial?
Our team of experts is always on hand to provide you with customized solutions. Feel free to contact us!
We let our work and our clients speak for us.
Request our guides now
